JOURNAL OF AMERICAN ACADEMY OF DERMATOLOGY (JAAD)
Objective outcome measures: Collecting meaningful data on alopecia areata. Bottom line: These methods will facilitate direct comparison of alopecia areata treatment outcome in both clinical practice and clinical trials.
Elise A. Olsen, MD, Janet Roberts, MD, Leonard Sperling, MD, Antonella Tosti, MD, Jerry Shapiro, MD, Amy McMichael, MD, Wilma Bergfeld, MD, Valerie Callender, MD, Paradi Mirmirani, MD, Ken Washenik, MD, PhD, David Whiting, MD, George Cotsarelis, MD, and Maria Hordinsky, MD
Background: Although alopecia areata is a common disorder, it has no US Food and Drug Administration approved treatment and evidence-based therapeutic data are lacking.
Objective: To develop guidelines for the diagnosis, evaluation, assessment, response criteria, and endpoints for alopecia areata.
Methods: Literature review and expert opinion of a group of dermatologists specializing in hair disorder.
Results: Standardized methods of assessing and tracking hair loss and growth, including new scoring techniques, response criteria, and end points in alopecia areata are presented.
Limitations: The additional time to perform the assessments is the primary limitation to use of the methodology in clinical practice.
Conclusion: Use of these measures will facilitate collection of standardized outcome data on therapeutic agents used in alopecia areata both in clinical practice and in clinical trials.
Key words: ALODEX score; alopecia areata; assessment measures; outcome measures; response criteria;SALT score.
Summary: Currently, the only standardized method for assessment of alopecia areata is the Severity of Alopecia Tool (SALT) score. This article offers recommendations for standardized assessment and response criteria in patients with alopecia areata. These methods will facilitate direct comparison of alopecia areata treatment outcome in both clinical practice and clinical trials.
Topical Janus kinase inhibitors: A review of applications in dermatology. Bottom line: The true potential of topical JAK inhibitors for inflammatory dermatologic diseases requires further study.
Anna-Marie Hosking, BS, Margit Juhasz, MD, and Natasha Atanaskova Mesinkovska, MD, PhD
Background: Janus kinase (JAK) inhibitors have attracted attention for their role in treating inflammatory disorders. This new class of biologics has the potential to significantly affect the field of dermatology, especially with the development of topical formulations.
Objective: To summarize published evidence on the efficacy, safety, and tolerability of topical JAK inhibitors in the treatment of inflammatory skin conditions.
Methods: This is a review of articles available in PubMed and the Cochrane Library up until November 2017.
Results: Fifty-five potential articles were identified; 11 articles were included for review, comprising an aggregate of 924 patients. In randomized clinical trials, topical JAK inhibitors demonstrate modest improvements in psoriasis and atopic dermatitis disease scores, patient-reported outcomes, and quality of life. Results for vitiligo are conflicting, with improvements seen only in facial vitiligo. Conclusive efficacy data for alopecia areata is lacking.
Limitations: It was not possible to perform a meta-analysis due to the lack of standardization and low number of randomized clinical trials.
Conclusion: Topical JAK inhibitors provide an attractive treatment option for patients with psoriasis, atopic dermatitis, alopecia areata, and vitiligo. Although early phase clinical studies of this novel drug class are promising, large phase 3 and 4 studies are needed to further define the role of topical JAK inhibitors in dermatology.
Key words: alopecia areata; atopic dermatitis; JAK inhibitor; JAK-STAT; psoriasis; topical; vitiligo.
Alopecia areata is associated with impaired health-related quality of life: A survey of affected adults and children and their families
Lucy Y. Liu, MD, Brett A. King, MD, PhD, and Brittany G. Craiglow, MD
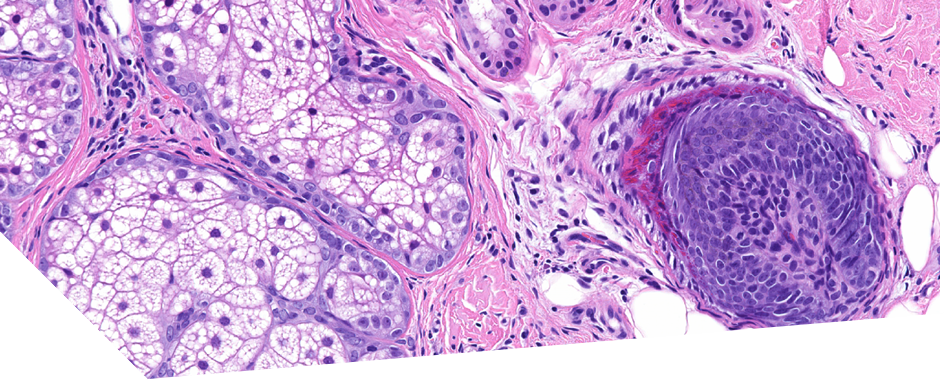
A technique for more precise distinction between catagen and telogen human hair follicles ex vivo
Irene Hernandez, PhD, Majid Alam, PhD, Christopher Platt, PhD, Jonathan Hardman, PhD, Eleanor Smart, MSc, Enrique Poblet, MD, Marta Bertolini, PhD, Ralf Paus, MD, and Francisco Jimenez, MD
Whole-exome sequencing reveals differences between nail apparatus melanoma and acral melanoma
Minho Lee, PhD, Jonghwan Yoon, BS, Yeun-Jun Chung, MD, PhD, Soo Young Lee, MD, Jin Young Choi, MD, Ok Ran Shin, MD, PhD, Ho Youn Park, MD, Won-Jong Bahk, MD, PhD, Dong Soo Yu, MD, PhD, and Young Bok Lee, MD, PhD
Clinical, dermoscopic, and trichoscopic analysis of frontal fibrosing alopecia associated with acquired dermal macular hyperpigmentation: A cross sectional observational case-control study
Muthu Sendhil Kumaran, MD, DNB, MNAMS, Muhammed Razmi T, MD, DNB, Keshavamurthy Vinay, MD, DNB, MNAMS, and Davinder Parsad, MD
JOURNAL OF INVESTIGATIVE DERMATOLOGY (JID)
JAK Inhibitors for Treatment of Alopecia Areata
EHC Wang, BN Sallee, CI Tejeda and AM Christiano
This review summarises and discusses the current pre-clinical and clinical studies on JAK inhibitors, as well as the prospects of using JAK inhibitors for the treatment of alopecia areata.
BRITISH JOURNAL OF DERMATOLOGY (BJD)
Is methotrexate an effective and safe treatment for maintaining hair regrowth in people with alopecia totalis? A Critically Appraised Topic*
R. Browne L. Stewart H.C. Williams
Clinical question/scenario: Is methotrexate (MTX) an effective and safe treatment for maintaining hair regrowth in people with alopecia totalis?
Background: Alopecia areata (AA) is a common disorder causing non scarring hair loss with an estimated lifetime prevalence of approximately 2%. Treatment of extensive AA is challenging. The aim of this Critically Appraised Topic was to assess the current evidence regarding use of MTX for inducing and maintaining hair growth in patients with alopecia totalis.
Methods: We critically appraised the literature identified from searching PubMed, Ovid MEDLINE, Ovid Embase and Cochrane Central (October 2017), using the search terms (“alopecia areata” OR “alopecia totalis” OR “alopecia universalis”) AND (methotrexate).
Results/identified evidence: Two prospective studies and 11 retrospective case series were included, comprising 226 patients with alopecia varying from 30% hair loss to alopecia universalis at baseline. MTX was usually given with systemic corticosteroids for induction of hair regrowth rather than regrowth maintenance. Regrowth, defined as anything from 50% to complete regrowth, was reported in 20–90% of patients. Relapse occurred in 20–80%, with variable regrowth on retreatment. Most series were small, with limited methodological detail and follow‐up data. Adverse effects ranged from 7% to 60%.
Discussion and recommendation for clinical case: We found insufficient evidence to conclude whether MTX is useful for maintaining regrowth in extensive AA. We found some evidence to suggest that hair regrowth may be induced by MTX when used in combination with systemic corticosteroids, but it was difficult to attribute responses to any one treatment or spontaneous regrowth. Included case series were at a high risk of bias. Randomized controlled trials are needed to evaluate whether MTX alone, or in combination with corticosteroids, vs. placebo is useful for inducing and/or maintaining remission of hair regrowth. In the meantime, MTX may occasionally be considered in people with severe disease that significantly impacts on their quality of life.
CLINICAL AND EXPERIMENTAL DERMATOLOGY (CED)
Risk factors associated with frontal fibrosing alopecia: a multicentre case–control study
O. M. Moreno‐Arrones D. Saceda‐Corralo A. R. Rodrigues‐Barata M. Castellanos‐González M. A. Pugnaire R. Grimalt A. Hermosa‐Gelbard C. Bernárdez A. M. Molina‐Ruiz …
Background: Frontal fibrosing alopecia (FFA) is a chronic cicatricial alopecia with an increasing incidence and unknown etiology.
Aim: To identify possible environmental and hormonal factors related to FFA.
Methods: We conducted a multicentre case–control study paired by sex and age, and recruited 664 women (335 cases and 329 controls) and 106 men (20 cases and 86 controls). Study subjects completed an exhaustive questionnaire enquiring about pharmacological, environmental, hormonal, social, job exposure, lifestyle, drugs and diet factors to which they were exposed at least 5 years prior to the onset of the disease.
Results: For women, there was a statistical association between alopecia and history of pregnancy (OR = 1.6; 95% CI 1.06–2.41), use of facial sunscreen (OR = 1.6; 95% CI 1.06–2.41) and hormone replacement therapy (HRT) (OR = 1.76; 95% CI 1.11–2.8) or raloxifene (no controls exposed therefore OR was not calculated), exposure to alkylphenolic compounds (OR = 1.48; 95% CI 1.05–2.08), and presence of rosacea (OR = 1.91; 95% CI 1.07–3.39), lichen planus pigmentosus (LPP) (OR = 5.14; 95% CI 1.11–23.6) or hypothyroidism (OR = 1.73; 95% CI 1.11–2.69). For men, there was a statistical association between alopecia and use of facial sunscreens (OR = 11.6; 95% CI 1.7–80.9) or anti ageing creams (OR = 1.84; 95% CI 1.04–3.23).
Conclusions: FFA seems to be associated with hormonal exposure (pregnancy, HRT and raloxifene), comorbidities (hypothyroidism, LPP and rosacea) and environmental factors (facial sunscreens, anti ageing creams and occupational exposure). Further research is required to analyze the exact mechanism in which these environmental factors participate in the development of this alopecia.
Increased risk of severe course of pemphigus in patients with pemphigus‐associated alopecia: a prospective observational study
M. Sar‐Pomian J. Czuwara L. Rudnicka M. Olszewska
Background: Pemphigus‐associated alopecia is considered rare, and has not been studied in detail.
Aim: To evaluate the clinical and immunological characteristics of patients with pemphigus‐associated alopecia.
Methods: This prospective observational study included 80 consecutive patients with histopathologically and immunopathologically confirmed pemphigus, of whom 11 (13.8%) were found to have pemphigus‐associated alopecia. Alopecia was observed in 11/52 patients with pemphigus and scalp involvement: [0/28 (35.7%) with pemphigus vulgaris and 1/24 (4.2%) with pemphigus foliaceus. The clinical and immunological characteristics of these patients were analyzed.
Results: Patients with pemphigus‐associated alopecia had a significantly higher Pemphigus Disease Area Index total activity score compared with patients who had no pemphigus‐associated alopecia (21.8 ± 18.6 and 11.0 ± 20.5, respectively; P = 0.02). Mean serum anti‐desmoglein (Dsg) 1 antibody concentration was 141.8 ± 66.9 U/mL and 60.0 ± 52.6 U/mL, respectively (P = 0.03), and mean serum anti‐Dsg3 concentration was 126.6 ± 36.7 U/mL and 67.4 ± 52.5 U/mL, respectively (P = 0.03). The values for achieving serological remission were 10% and 70%, respectively (P = 0.02).
Conclusions: Pemphigus‐associated alopecia is a marker of severe disease and a treatment‐resistant disease course.